|
|
| << PREV |
|
|
Biology takes her time from geology. The only
reason we have for believing in the slow rate of
the change in living forms is the fact that they
persist through a series of deposits which geology
informs us have taken a long while to make. If
the geological clock is wrong all the naturalist
will have to do is to modify his notions of the
rapidity of change accordingly.
THOMAS H. HUXLEY
(1869, 25:xlviii)[1]
There is no question that technology has made tremendous advances in the past few decades while, in contrast, science is still laboring under the highly questionable Lyellian dogma of uniformitarianism. More specifically, advances in technology have enabled radiometric results to be obtained that, by uniformitarian interpretation, appear to offer evidence supporting the evolutionary requirements for an old earth. Textbook descriptions of these highly technological methods are given in generalizations that leave the reader convinced that the radiometric dating methods have long been perfected and now provide absolutely reliable results. For example, not too many decades ago the radiometric ages of fossil artifacts were often reported with plus and minus tolerance values that gave a false impression of integrity and precision. What is often overlooked is that the tolerance figures apply only to the technology of the method and have no bearing on the underlying assumptions of science. These assumptions were listed in the previous chapter together with some suggestion that the decay constants may not have been as constant as it has been assumed. In this chapter, further evidence is given that puts into serious question all the remaining assumptions related to the origin of the minerals on which radiometric determinations are conducted.
It was pointed out earlier, and it will be worthwhile to mention again, that in matters of time in the remote past, that is, prior to human records, there can be no absolute proof of the duration of time. Once datable human records begin to appear, roughly two thousand years ago, it then becomes possible to acquire independent confirmation of radiometric dates; in this case only the carbon 14 radiometric method qualifies, for reasons that will be made clear later in this chapter. For time periods believed to be hundreds of millions of years in the past, it is a matter of making assumptions rather than having faith in what is seen as solid evidence.
The radiometric methods appear to offer evidence for an old earth, but
there are many phenomena that cannot be explained in terms of the evolutionary
long ages; seldom are the implications or even the phenomena themselves
mentioned in the published media. A few examples of evidence that indicates
a young earth are given in the remainder of this chapter. It is only by
considering a balanced sample of evidence that any meaningful judgment
can be made to accept the validity of claims made for the age of the earth.
Enigma in the Basement Rocks
The principal assumptions associated with radiometric dating, which were listed earlier, began by presupposing that the earth originated from a spinning blob of hot liquid that cooled to form the crustal material. It is further supposed by current theory that the ravages of time preclude the survival of the original crustal material. All that is believed to remain today are the igneous rocks that have crystallized from hot liquid magma, long after the original cooling, together with the sedimentary rocks that have originated by erosion and redeposi-tion. The igneous rocks are, essentially, the granites, and these form the basement material underlying all the layered sedimentary rocks. Often there are thousands of feet of sedimentary rock on top of the basement material, but, exceptionally, this basement rock is found at the surface, as it is over a large part of Canada, where it is known as the Canadian Shield.
Almost a century ago microscopic studies of this basement rock, taken from various parts of the world, revealed small concentric circles of discoloration associated with certain minerals (chiefly mica) within the rock matrix. These tiny, colored, circular rings are really the sections through spheres having a small inclusion at the center; they were at first called "pleochroic halos" but are now usually referred to as "radio-halos" (Joly 1917).[2] It was not until a few decades ago that the halos were recognized to be the "signatures" of the radioactive products of the uranium 238 decay series.
When an inclusion of uranium 238 in the mineral crystal lattice begins to decay, alpha particles (which finish up as helium atoms) or beta particles (electrons), depending on the stage of decay, are projected out in all directions at high speed and travel through the surrounding material. At each specific stage of decay, these particles all have the same energy, and all penetrate identical distances, leaving an abrupt edge to the sphere that appears as a circle when precisely sectioned. The circle diameter is, therefore, directly related to the particular energy of the projected particle, and since this is different for each stage in the decay series, the circle diameter becomes a "signature" of the individual decay process. The series of concentric circles is as sure as a fingerprint in identifying the decay process. There are actually fourteen stages in the decay of uranium 238 to lead 206, and these are given fully in Appendix C. However, one of the most common "signatures", found by the million throughout the basement rocks, is that of polonium 218 which occurs about midway through the overall uranium 238 decay process and has a half-life of only 3.05 minutes.
Robert Gentry (1974) is acknowledged to be the foremost expert in the field of radio-halos. By the use of the ion microprobe, he has been able to analyze the microscopic inclusions at the center of the concentric circles. This modern device was not available to early investigators and enables the identification of individual atoms, and also permits them to be counted in order to establish the relative abundance of each element present. Gentry's investigation of the commonly found polonium 218 halos by microprobe analysis has shown that the inclusion at the center consists mostly of the final product, lead 206. The startling thing is that there are no elements above polonium in the inclusion; in other words, the daughter elements are present but no parents (see Appendix C). When it is recalled that the half-life of the parent uranium 238 is said to be 4.5 billion years, then a little more than half the original quantity of uranium would be expected to be present. In fact, not one atom of uranium or thorium can be found, nor are there any traces of the characteristic halos for these elements.
There is no doubt that the halo "signatures" are genuinely those of part of the uranium 238 decay series. Even if the velocity of light and the related speed of particle emission had been radically different in the past, this would not, it has been pointed out by Setterfield, affect the halo diameters. This is because the electron rest mass was lower in the past (Appendix E), and the specific electron charge was higher (Appendix F); the differences thus cancel out and leave the penetration distance unchanged (Steidl 1982).
The simple evidence of the "daughter" elements without a trace of the "parent" leaves one little choice but to conclude that the decay process began with polonium 218. However, since the half-life of this element, even measured today, is only 3.05 minutes, it could not have begun in the liquid state. The reason for this is that all the alpha particles were emitted from the decaying polonium in the first hour or so and if emitted within a liquid medium, would leave no permanent record. This forces the conclusion that the polonium decay began in the solid state; we are faced here with evidence of the original Creation. If this is true, and no other rational explanation is yet forthcoming, then it means that all the basement rocks were supernaturally created in the solid form and never passed through the liquid to solid change by slow cooling. Gentry put the matter this way: "Is it conceivable that one of the oldest cosmological theories known to man [biblical Creation] is correct after all? Could the earth have been created by fiat?" (Gentry 1967, 78).[3]
The scientific community acknowledges that Gentry's work has been most
thoroughly and carefully carried out, yet it is extremely reluctant to
draw these conclusions from the evidence, because it would at once invalidate
all the assumptions concerning the earth's origin and those basic to the
radiometric methods.
The Appearance of Age
The evidence of the polonium radio-halos is, seemingly, evidence for
ex
nihilo creation -- instant creation of something out of nothing. This
is plainly a supernatural phenomenon and, to many minds, a major stumbling
block. Yet what alternative explanation does the evolutionary scenario
have to offer? In the introduction, Harlow Shapley was quoted as the representative
of today's explanation for the origin of the universe, and his statement
may be paraphrased, "...in the beginning hydrogen" (Shapley 1960, 3). However,
when this is considered, it surely involves ex nihilo creation of
hydrogen atoms from nothing and, as such, is no less supernatural than
ex
nihilo creation of solid rock containing polonium halos. So far as
this author is aware, no other explanation has ever been proposed to explain
the initial appearance of all things. Ex nihilo creation admittedly
offers little intellectual satisfaction, in terms of today's scientific
mind-set, but even to accept this as a theory would seem better than having
no theory at all. If the concept of a vast age for the universe is found
to be based on assumptions and if there is good contrary evidence that
indicates a young earth, then with a drastically shorter time frame, the
initial appearance of matter could not have begun with hydrogen but must
have begun with the universe, more or less the way it is today. The planet
earth would have been created with the instant appearance of basement rocks,
sand, topsoil, and all forms of life. This raises the question, If the
initial life forms were created, how old did they appear to be at the first
moment? It would be reasonable to say that the chicken appeared before
the egg, and that being so, the chicken may have had the appearance of
being, perhaps, one year old. The first man may have appeared to be, perhaps,
thirty years old; hardwood trees, one hundred years old; and coral reefs
large enough for fish to live in -- perhaps several thousand years old.
All this appearance of age is then a necessary part of ex nihilo
creation,
once this concept is accepted.
Carbon 14 Dating
One of the most spectacular sights to be seen in the night sky is the beautiful aurora borealis, known in the north as the northern lights and seen best in the extreme northern or southern latitudes. These appear as curtains or streamers of colored lights, very high in the atmosphere and stretching in an east-west direction, while slowly moving as a band across the sky towards the magnetic pole (Roble 1977). The lights are the result of the ionization of upper atmosphere atoms by cosmic rays and is the same type of effect as that produced by the applied voltage to neon gas in electric signs. Cosmic rays are extremely high energy particles that originate somewhere in outer space; their source is still uncertain (Rosen 1968). As the earth travels through space, it crosses the path of millions of these particles, which are similar to X rays and are known to cause genetic damage to reproductive cells and which, subsequently, result in birth defects. For this reason, there has always been concern about their effect on astronauts. Fortunately, for life on earth there are two protective barriers that prevent most of these harmful rays from reaching the earth's surface. The first is the earth's magnetic field, which extends into space and acts as a shield guiding any cosmic particles encountered towards the north and south poles. These potentially lethal areas are, in any case, inhospitable to life.
The second line of defense is the earth's atmosphere, filled with gaseous atoms, more than 70 percent of which are nitrogen, the remainder being mostly oxygen. A small residual percentage consists of helium and argon atoms, some molecules of water, carbon dioxide, ozone and, more recently, molecules that cause the acid rain problems. Bearing in mind that atoms and atom combinations (molecules) are mostly empty space, those high-speed cosmic particles that get past the magnetic barrier tend to pass right through many of the atmospheric atoms. When they eventually hit the nucleus of a gaseous atom, they release a neutron; the atom then becomes ionized. It is mostly nitrogen atoms that then capture the free neutrons, with the result that these stable nitrogen 14 atoms become unstable carbon 14 atoms. The number refers to the atomic weight or mass. Once formed, the radioactive C14 atoms begin to decay by emitting beta particles (electrons) and revert back to stable nitrogen 14 atoms once more.
The C14 atoms are comparatively rare since for every one of these there are 765 billion normal, stable C12 atoms. One of the important assumptions made is that this ratio of C14 to C12 (which has been determined in recent years) has been constant for at least the past fifty thousand years. This assumption is, in turn, based on the uniformitarian assumption that C14 production and decay has been going on for millions of years and long ago reached equilibrium; it is further assumed that perfect atmospheric mixing has been achieved, so that the ratio of C14 to C12 is the same everywhere. Immediately after formation in the atmosphere, the C14 atom is joined by two oxygen atoms to become a molecule of carbon dioxide, and together with all the other carbon-dioxide molecules containing the stable C12 atoms, becomes part of the great carbon cycle of life.
The carbon cycle is simply that process in which the carbon dioxide from the atmosphere is absorbed by the leaves of plants and, by the process of photosynthesis, is converted into sugars. The plants are part of the food chain for animal life, in which some carbon in the sugar is converted to carbonates for bone, etc. While most of the carbonates contain the stable C12 atom, some contain the unstable C14 atom; it is assumed that during life this ratio of C12 to C14 was the same as that in the atmosphere. In certain cases, however, this has since been found not to be true. When the food intake ceases upon the death of the organism, the ratio of C12 to C14 begins to change as the unstable C14 decays to nitrogen 14; the gaseous nitrogen escapes to the atmosphere. It is at this point that the C14 clock begins to measure time. The decay rate of C14 is assumed to have been constant throughout the ages, so that by finding this rate and the quantity of C14 remaining in the material being dated, it is, theoretically, possible to find its age.
Willard Libby, working at the University of California, developed the
C14 dating method in 1947 and subsequently received the Nobel
prize for his work. He found the nuclear decay constant and reported the
mathematically related half-life for radioactive C14 as 5,550
years (1947 figure). The extremely small proportion of C14 to
C12 in the atmosphere, and subsequently in the living organism,
becomes even smaller as the C14 decreases with the length of
time between death and analysis. For example, on the basis of Libby's original
half-life, if the organism died containing, for example, one hundred C14
atoms, then after six half-lives, that is, 33,000 years, there would be
less than two c14 atoms left. This presented an upper limit
of about 50,000 years, beyond which the number of C14 atoms
remaining would be too few to be detectable by the method. Even so, relatively
large samples of one hundred grams (four ounces) were required and destroyed
in the test (Libby 1955). Litherland (1980) describes a new high-energy
method that has been developed enabling much smaller samples to be used
and extending the time limitation somewhat beyond 50,000 years. The underlying
assumptions for the C14 method, however, remain the same.
Carbon 14 Results
It was in the early fifties that archaeologists and geologists adopted the method as acceptable, even placing it above traditional methods of dating and sweeping aside physical evidence that showed the C14 results to be in error. Lee says that "radiocarbon swept the scientific world with all the fervor of religious fanaticism, as the new and 'absolute' chronology was established" (Lee 1981, 9). In those early days the method was applied to almost anything containing carbon, and the results were published in the newly formed Radiocarbon Journal, a kind of clearing house for C14 data from all the various laboratories. Hundreds of fossil bones of Neanderthals, Cro-magnons, mammoths, sabre-tooth tigers, and other extinct animals, as well as fossil trees, coal, oil, and natural gas, were all reported having ages, by the C14 method, of only several thousand years. The significant point is that every biological specimen tested contained C14, and all appeared to lie within a 50,000-year time frame; a selection of some of these reported values is given in Appendix J. The great number of these results, indicating a young age for material in some cases believed to be millions of years old, had disturbing implications for the geological time scale; using the oxygen 18 method one outcome was that the long-accepted estimate of the time of the last ice age was cut down by half, to 11,000 years ago (Emiliani 1956; Knopf 1957, 233).[4]
These early workers were often physicists, and, perhaps somewhat naive to the prejudice of the establishment, many of them simply reported what they found. This is honest science carried out according to Baconian principles. In more recent years, C14 dates on such items as coal, oil, or dinosaur bones no longer appear in Radiocarbon Journal, because by now it has been impressed on research workers from their student years that the C14 method does not give results with materials "known" to be older than about 50,000 years; this is clearly untrue as shown by the early published results. The public, which ultimately pays for all this research, is generally quite unaware of the unbelievable circularity in the procedure for submitting samples to laboratories for C14 analysis. The investigator is first asked what date he will accept; then, when a figure is obtained that comes near this date, it is duly reported together with the tolerance value, and these figures become sacrosanct, reported in journal after journal, year after year. Ogden, the director of a radiocarbon laboratory, has made the remarkable confession: "It may come as a shock to some, but fewer than 50 percent of the radiocarbon dates from geological and archaeological samples in northeastern North America have been adopted as 'acceptable' by investigators" (Ogden 1977, 173). Clearly, the C14 method of dating, as with the other radiometric methods, is either reliable and useful or it is not.
In the last two decades some concern has been expressed for the usefulness
of the C14 test method. Techniques have improved, but still
there are uncertainties and absurd results, not with old material that
appears young, for which there is no proof of age, but for recent material
that appears old, for which there is proof. Living mollusk shells have
been dated by the C14 method at up to 2,300 years, a freshly
killed seal at 1,300 years,[5]
and wood from a growing tree at 10,000 years (Dort 1971; Huber 1958; Keith
and Anderson 1963). Whenever it can be justified, unexpected figures are
adjusted up or down, according to the need, on the basis of a whole list
of factors that are believed to have either added C14 atoms
to the specimen if it appears too young or received too little C14
in the first place if it appears too old. For instance, the new high-energy
mass spectrometry method (AMS method), previously mentioned and involving
a count of individual atoms, was delayed for some time because the ages
of the samples consistently came out too young (Grootes 1980).[6]
With the new technology, these were probably the true results, but they
were found unacceptable because they did not reconcile with all the previous
selected results and, ultimately, with Lyell's geology; the research workers
were then forced to conclude that the young ages were due to an unknown
source of C14 somewhere in the equipment! None of this is ever
mentioned in popular magazines and textbooks, and the impression is left
in the reader's mind that "absolute" chronology has been established by
the radiocarbon method.
The Underlying Assumptions
The assumptions that were made when the C14 method was established in the early 1950s are summarized below; subsequent problems, however, have caused some of these assumptions to be modified, as will be explained later.
1. It is assumed that the rate of production of C14 from nitrogen has been the same in the past as it is today. This includes the assumption that the rate of cosmic ray bombardment and the magnetic and atmospheric barriers that provide protection have always been the same (Kulp 1952, 261).[7]
2. It is assumed that the C14 to C12 ratio reached equilibrium millions of years ago, and that during the course of this time there has been plenty of atmospheric mixing to give a uniform distribution. This may be recognized as a tautology, since what is really being implied is that, because the atmospheric system has been in existence millions of years, it must have reached equilibrium, and because it is in equilibrium, it must have been in existence for millions of years (Suess 1965, 5947).[8]
3. It is assumed (from assumption 2 above) that every living organism contained at death the same C14 to C12 ratio as is found in the atmosphere today.
4. It is assumed that the artifact being dated has been a closed system -- that is, there has been no C14 loss other than by decay and no c14 addition during the period between death and analysis. However, this assumption can be overridden by making appeal to a wide range of causes assumed to have added or removed C14 after death in order to adjust the initial figures to those expected.
5. It is assumed that the measured specific rate of sixteen counts
per gram per minute for C14 decay has been the same in the past.
In other words, the related half-life of C14 is assumed to
have always had the same value.
A Closer Look at Some of the Assumptions
Libby, in his original work in the late 1940s, found that the specific production rate of C14 in the upper atmosphere then was 18.8 atoms per gram per minute; details of how this measurement was made have been given by Libby (1955, 7) while the qualifying word "specific" relates to the units being expressed as "per gram" and simplifies the comparison of rates (these terms are explained in note 14 of Chapter Eleven). Libby then took samples of wood from Pharaoh's tomb and other carbon-containing samples of known age from many parts of the earth and found the specific decay rate of C14 very close to average 16 atoms per gram per minute. Now it may be seen that these figures for production and decay are not the same; in fact, there is almost a 20 percent difference, but Libby (1955) reconciled this by his statement: "The agreement seems to be sufficiently within the experimental errors involved so that we have reason for confidence in the theoretical picture" (Libby 1955, 7). The theoretical picture to which he refers is firmly rooted in the doctrines of uniformitarianism, which demands equilibrium between production and decay rates. To acknowledge any difference in the rates would allow the data to point to a beginning, which, in this case, would be relatively recent.
Now that the C14 dating method has been established, but essentially confined to the biosphere (samples of less than 50,000 years), statements are beginning to appear in textbooks that acknowledge that the C14 decay rate in living organisms is about 30 percent less than its production rate in the atmosphere.[9] Stansfield (1977, 83), in a recent textbook, even admits that from this difference in rates it can be argued that the age of the atmosphere is less than 20,000 years old. The evidence would seem to indicate that this is the case, while from the figures it may be appreciated that any increase in the difference between rates of production and decay will shorten the age of the atmosphere still further. Whatever the result may finally be, an age of thousands of years is far removed from the hundreds of millions required by Darwin. With this acknowledgment of a difference in rates, it is then reasonable to ask whether the production rate has increased while the decay rate remained constant, or whether both rates have changed. There is evidence that strongly suggests that both rates have changed with time, which not only indicates that the system must be relatively recent but also would shorten the ages found still further. First, however, we will see what has caused the C14 production rate to have increased throughout the centuries.
It was mentioned earlier that C14 atoms are produced in the upper atmosphere by the interaction of cosmic rays, and while the magnetic field of the earth provides a first line of defense against these powerful rays, it is known that the magnetic field has been decreasing at a rather alarming rate (Magsatdown 1980). With a greater field strength in the past, fewer cosmic particles would have entered the atmosphere, and fewer C14 atoms be produced. This would mean -- under the reasonable assumption that the organisms that died in the past would retain the same C14 to C12 ratio as in the atmosphere at that time -- less C14 to begin with at the time of death. After a further reduction in the C14 content by decay, the sample, when measured today, would thereby appear to be a great deal older than is actually the case.
The second line of defense against cosmic rays is the atmosphere itself or, more specifically, water vapor in the atmosphere. Dillow has developed the vapor canopy theory which proposed that the early earth was completely protected from cosmic rays by a shell of invisible water vapor high above the atmosphere. The vapor canopy was also claimed to have provided a "greenhouse" effect for the primeval earth and caused the atmospheric pressure to be double what it is today (Dillow 1981, 146).[10] The vapor canopy theory is still popular but often overlooked is the fact that when water changes from vapor to liquid the latent heat of condensation is released. Calculations show that the heat given out at the collapse of such a canopy could present a serious problem to life on earth. For this reason, if the theory is correct, the amount of water precipitated could not amount to more than a couple of feet. Absence of cosmic rays in the atmosphere would mean that no C14 was produced at this time. We have already seen from the difference in the rates of production and decay that C14 production appeared to begin about 20,000 years ago. However, as we shall see later, with some further downward correction for the changing decay "constant," the beginning of C14 production would be brought to roughly 5,000 years ago. With the collapse and loss of the vapor canopy at this time, possibly by dust nucleation from volcanic activity, C14 would then begin to be produced and become part of the carbon cycle in the food chain. The time of this proposed event, the resultant effects in terms of a world-wide flood, and the subsequent genetic damage by secondary radiation (C14 decay within living tissue) would seem to be supported by the world-wide Flood traditions and the Genesis claim for the longevity of early man (Bjorksten 1963; Upton 1957).
While the former existence of a vapor canopy appears most unlikely, the thickness of the earth's atmosphere may have been greater in the past. Evidence for this is scanty and related to the fossils of enormous flying reptiles. The well-known pteranodon had a wingspan of up to seven meters (twenty-three feet) and experts have long debated whether the creature would have had sufficient muscular strength for powered flight. It was generally concluded that it must have lived on cliffs and sailed on the updrafts above the sea in search of fish (Langston 1981). However, a specimen of an even larger flying reptile, the pterosaur, with an estimated wingspan of fifteen meters (forty-nine feet), was reported by Lawson in 1975.[11] Under present day conditions this creature could never have left the ground. However, if the atmospheric pressure, i.e. the air density, was twice as great in the distant past, it does appear that the giant reptile could just have become air-borne (Bramwell and Whitfield 1976).
Finally, there is the question of the constancy of the nuclear decay constants for the radioactive elements, which was raised in the previous chapter. While published values of the half-lives of C14 do show an apparent increase from 5,568 years in 1955 to 5,770 in 1980, this has been brought about by an international agreement to make a correction for the man-made carbon dioxide and radiation introduced since 1850, and by the improved counting techniques (Stuiver and Suess 1966). However, the more complete counting of emitted particles would result in a shortening, rather than a lengthening, of the half-life, so that a real increase in the decay "constant" can be suspected.
Textbooks today recognize that correction factors are necessary to take into account the disequilibrium between C14 production and decay. Figures of 25 percent reduction in age for an uncorrected age of 10,000 years are quoted, while greater reductions would apply to material that appears older. Cook proposes even greater corrections of 20 percent for 1,000 years, 30 percent for 4,000 years, and so on, which would telescope all the long C14 ages to 12,500 years or less (Cook 1966, 8). If, in addition to this correction for disequilibrium between C14 production and decay, a further downward correction is made for a decrease in the decay constant, the radiocarbon method begins to produce ages all within a time frame of a few thousand years.
Whitelaw (1970) has subjected 15,000 published C14 dates
to statistical analysis by ranking, and then has applied the correction
factors using the acknowledged 30 percent difference in rates, and the
entire data reduce to a remarkably sharp beginning point, about 5,000 years
ago. This, again, is confirmation of the Genesis record for the time of
the Flood and a good reason to question openly all the long ages given
by the other radiometric methods, reckonings we have been assured are based
on sound scientific principles.
What Can be Concluded About Radiometric Dating?
Very seldom is the word "calibration" mentioned in popular or even textbook explanations of radiometric dating, possibly because it is one of the weakest areas in the whole exercise. It is normal procedure in any analytical laboratory to calibrate the test method against a known standard before attempting analysis on the unknown sample. It is important that the age of the known standard, or primary calibration standard, has been determined by an entirely independent physical method; with the majority of radiometric tests this is, of course, quite impossible. Libby (1963) used archaeologically dated wood from Pharaoh's tomb for the early radiocarbon calibration, but since several ounces of sample were required and the test is destructive, calibrations of this type are, obviously, very limited (See Chapter Thirteen, note 9). Recognizing this limitation, attention was then given to the bristlecone pine tree (Pinus aristata) and the giant redwood trees (Sequoia gigantea), which are among the oldest living things; Libby (1963, 279) thought they could be accurately dated by counting the rings. However, after a great deal of work it was discovered that these trees could add more than one ring a year, and this has evidently led to some inconsistencies (Clock and Agerter 1963; Jueneman 1972).[12] In any event, whether by using wood from Egypt or from the pine trees, this calibration material is only good for little more than three thousand years, but beyond this time there is still a real need for a good independent method of finding the age of carbon-containing material.
Lee, writing in the Anthropological Journal of Canada, makes
the statement: "The necessity for calibration over the last 7000 years
is well recognized and attended to, while the probable error in older dates
receives no practical consideration at all. At a range of 20,000 to 30,000
years, it is true, one can only guess at the full extent of the problem.
But one can be reasonably sure about its trend: too young" (Lee
1981, 25. Emphasis in original). This is a continuing problem among the
radiocarbon fraternity, where there seems to be an ongoing search for reasons
to increase the ages, while all the hard evidence keeps pointing in the
opposite direction. Some investigators have become quite irritated, and
Lee (1981, 27) sums up Stuckenrath:
Radiocarbon method is still not capable of yielding accurate and reliable results. There are gross discrepancies, the chronology is uneven and relative, and the accepted dates are actually selected dates. "This whole blessed thing is nothing but 13th century alchemy, and it all depends upon which funny paper you read" (Stuckenrath 1977, 188).
This statement, by a worker in the field, sums up the truth of the
matter -- a far cry from the textbook claims of the "consistency of radiocarbon
dates".
We may reasonably conclude that within the dating range of calibration standards, perhaps the past five thousand years, the carbon 14 method is probably a good indicator of true age, especially when carried out by the new high-energy technique. For material believed to be older than this, however, the results obtained are all subject to interpretation, according to the presuppositions of the investigator, and the exercise then passes from the area of true science into that of pseudoscience.
When it comes to the other radiometric methods, such as the potassium/argon,
there are no independent test methods; thus there can be no primary calibration
standards. The use of fossils to calibrate the radiometric method, meanwhile,
is simply adding to an already circular situation. Any consistency found
with various radiometric methods is simply consistency within test methods
based on the radio-decay phenomenon and, as we have seen, these are all
subject to the same assumptions. The acceptance of the extreme ages given
by these radiometric methods is, therefore, not based on good science but
rather on philosophical grounds, because they appear to give support to
Lyell's geology.
Evidence That Demands a Verdict
The expanded time frame for the age of the earth is the central foundation stone for today's theory of evolution while the evidence for these long ages is provided, not by the C14 dating method, but by the other radiometric methods. The assumptions underlying these methods are crucial, and it is for this reason that some time has been spent bringing these into the light of day.
While it is quite unlikely that the exact age of the earth will ever
be known, there is an impressive number of quite unrelated natural processes
that indicate that the earth is less than one million years old; indeed,
many of these indicate that it is less than 100,000 years old. In either
event, these times are far too short for evolution to have taken place.
The remainder of this chapter will present, quite briefly, some of these
natural processes for which orthodox science has no satisfactory explanation
but which can be readily explained by a young earth.

Hermann von Helmholtz, 1821-94. In a very readable little paper (1856), this German physicist showed that the sun's constant output of energy could most readily be explained by contraction under its own gravitational field. (Metropolitan Toronto Reference Library Board) |
The Sun's Source of Energy
It has long been a cause for wonder how it is possible for the sun to keep pouring out enormous quantities of energy, of which the earth receives less than a billionth part, year in and year out, without any apparent signs of change. The problem is compounded by the fact that if the sun had been even slightly hotter or cooler in the past, then life on earth would not have been possible at all. The virtual constancy of heat output over the millions of years alleged for life to have evolved either has a rational explanation, or it is one of the miracles of evolution. The German physicist Hermann von Helmholtz proposed a rational explanation, in 1856, and said that the sun was shrinking under its own gravitational force; it was the contraction that provided the constant source of energy (Helmholtz 1856, 506).[13] This explanation allowed a maximum possible age for the sun of about ten million years, which was all very well for the time prior to Darwin's Origin but began to fall badly short of all the time needed by Darwin and his followers. The theory of the shrinking sun was quietly abandoned, leaving no other explanation. Then, in 1903, George Darwin, son of Charles, suggested that radioactivity, such as produced by radium, might be the source of the sun's heat, and within a week the idea was supported by others who could see this as an explanation for the greater age required by Darwinian evolution. As nuclear forces came to be understood in the 1920s, Sir Arthur Eddington (1926) then proposed that the sun's heat was produced by thermonuclear reactions, and, essentially, this has been the neatly pigeon-holed explanation to this day. The thermonuclear source of energy serves not only for our sun but for every star in the universe, but it is a theory held to more by faith than by fact. |
| Nuclear fusion processes, similar to that of the hydrogen bomb, produce subatomic particles called neutrinos. It was expected that the earth would be bathed in these particles as they continuously pour out of the sun's interior. After a number of elaborate experiments conducted by Bahcall (1969), however, the quantity of neutrinos detected was "less than a fifth of the predicted value and may be zero" (Yockey 1977; Bahcall and Davis 1976).[14] This leaves the theoreticians in a dilemma; indeed it has been admitted by two workers that the "situation has advanced in the past years from being merely difficult to understand to being impossible to live with" (Trimble and Reines 1973). The solar neutrino problem is not confined to our planetary system but has cosmological implications. If thermonuclear reactions do not provide the sun's energy and contraction is discounted because the process can only account for a few million years, then the whole of astronomical evolution faces a serious challenge (Sutton 1980). |
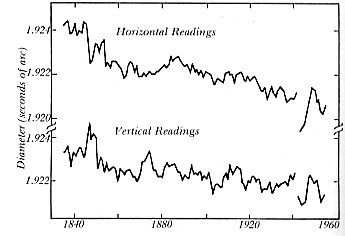
Contraction of the sun from Greenwich Observatory data. (After a diagram in Physics Today; author) |
Possibly because of the difficulty with the missing neutrinos, there
has been in recent years a renewal of interest in the solar contraction
theory, and once again the camp has been divided between those who accept,
and those who oppose the facts. Astrophysicist Eddy and mathematician Boornazian
(1979) analyzed solar measurements made regularly from 1836 to 1953 at
the Greenwich Observatory and found a statistically significant decrease
in the sun's diameter that exceeded errors of observation and even observer
bias. The same effect had been noted by others (Wittmann 1980).[15]
The rate of contraction amounted to about 0.1 percent per century, which
is surprisingly high and greater than that proposed by Helmholtz in 1854.
The objections to these findings came from two directions. Parkin son (1980)
made out an apparently convincing case by arguing that there was bias among
a series of observers, and he juggled the statistics to show random variation
but no contraction.[16]
Stephenson (1982), on the other hand, accepted the data as valid and the
decrease as real, but then argued that the phenomenon is cyclical; that
is, the observation is part of an ongoing cycle of contraction and expansion.[17]
While there is not a shred of evidence or explanation for this proposal,
the underlying purpose would seem to be to ensure leaving an open-ended
time in the past. The central problem of modern theories that try to explain
the source of the sun's energy and its necessarily constant nature is the
assumption that the sun is billions of years old. Without this overriding
presupposition, the evidence may be allowed to speak for itself, and while
the observations on contraction are still being debated, they can easily
be accommodated into the creation scenario and can readily explain the
sun's source of energy.
Rotating Sun -- Rotating Earth
When Galileo turned his telescope on the sun in 1610, he discovered dark spots on its luminous surface. From their movement he deduced that the sun rotated on its own axis once about every twenty-seven days. Since Galileo's time, many observations have shown that the spots at the higher latitudes rotate more slowly than those spots at the equator. Since the sun is gas and not a solid, these differences in rates of rotation are possible, although perhaps unexpected, and have given rise to speculations concerning the sun's interior, which, of course, is not directly visible. Some have argued for a rapidly rotating core, suggesting one turn a day, which has led to controversy, since it could be argued perhaps more cogently that the core was rotating more slowly than the envelope. The truth of the matter is that no one knows. But the awkward fact remains that different rotation rates exist. Howard has pointed out that the solar "wind", consisting of solar particles, constantly streaming away into space from the rotating surface, "exert a dragging effect that is strong enough to stop the rotation of the convective zone in only one million years" (Howard 1975, 112). In addition to this, the friction between the layers of hot gases within the sun's interior tend to reduce the differences in their rates of rotation and would shorten the overall rotation time still further. Howard confesses that "a million years is a short time ... and we know the sun's surface layers cannot be decelerating [slowing down] that rapidly. If they were ... only a few hundred million years ago the sun would have been rotating so fast that it would have thrown off an appreciable fraction of its mass by centrifugal force" (Howard 1975, 112). The central problem here is the assumption that millions of years are involved, while this, in turn, has led to the further assumption that the sun's surface is not "decelerating that rapidly". Without the biological and geological demands for billions of years required for evolution, the astronomical sciences would be free to push ahead with real scientific investigation of the cosmos. In the case of the sun's rotation, for example, the plain facts, without any appeal to miracle, would indicate that the age of the sun is certainly less than a million years.
Of course, the earth also rotates about its own axis once each day, and with the introduction of atomic clocks in the early 1960s it became possible to measure the length of the day to the nearest billionth of a second. A telescope was sighted onto one of the fixed stars and the interval timed when the same star returned to the cross hairs. It became evident that the earth was slowing down, and the small daily difference, when allowed to accumulate, amounted to 0.005 of a second per year each year; this is the earth's rate of deceleration (Thwaites and Awbrey 1982).[18] A remarkably stable system, really, but, nevertheless, this slowing of rotation in the vacuum of space is caused principally by the moon's gravitational pull on the oceans and the subsequent dissipation of this energy as tidal friction.
It is a fact that by international agreement a "leap second" has been
added to the world's clocks every year since 1972 on 31 December (Fisher
1973). This second includes the 0.005 of a second due to deceleration,
and the remainder of the second is a "velocity" adjustment to bring the
atomic clocks in exact alignment with the rotation of the earth. So far
this gives us no indication of a young earth; in fact, at the present rate
of deceleration, it can be shown that 4.6 billion years ago, the "day"
would have been a modest fourteen hours long (Thwaites and Awbrey 1982,
19). As tidal friction is slowing down the earth's spin, other processes
tend to work in the opposite direction (Challinor 1971).[19]
There is, for instance, undeniable historical evidence from eclipse data
showing that this has happened; a change as simple as a one meter fall
in sea level would decrease the rate of deceleration from its present value
(Stephenson 1982, 183). However, these changes in the earth's rate of rotation
are miniscule in comparison with an additional "leap second", which, The
Astronomical Almanac
(1983) records, has been added every year in July
since 1981.[20] This
now amounts to two seconds a year for correction instead of one, and only
time will tell what future changes will be necessary. The sea levels certainly
have not risen significantly, so that the earth's deceleration rate cannot
be responsible for the extra second; the situation does cast a nagging
doubt that perhaps the atomic clocks are not so constant after all.
Icy Visitors From Space
Every few decades our attention is drawn to the night sky to watch and wonder at the latest comet, although those within the living memory have turned out to be rather disappointing, in spite of the dire portents of doomsday prophets. Nevertheless, there is by now a lot of information about these icy and infrequent visitors which have been aptly described as "dirty snowballs", since they consist mostly of frozen water and dust. What we see as the comet head and long tail is really the sunlight scattered by the fine dust left behind as the ice ball evaporates in the vacuum of space; evaporation only occurs as the comet swings into orbit towards the sun. The comet, for us to see it with the naked eye, has to contribute about ten tons of dust every second to the inner solar system. This output of dust is represented by a much larger amount of frozen water, which comes off the ice ball as a fountain in all directions and dissociates into its component parts. A comet is, thus, in a process of rapid decay, which may last several months while in its orbit around the sun, all the while leaving behind a trail of gases and dust millions of miles long. Comets are believed to be as old as the solar system, and of the six hundred or so comets that are known, about one hundred move in orbits with periods of less than two hundred years. Probably the best known is Halley's comet. The painstaking records of the Chinese show that Halley's comet has appeared twenty-nine times at intervals of seventy-six and seventy-seven years; the first well-observed passage was 239 B.C. and the most recent was a disappointing appearance in 1986. It is estimated that the icy ball inside the head of this comet is some five miles in diameter, and conservative estimates are that the comet loses a fraction of 1 percent of its total substance on each return (Whipple 1974).
It should be evident from these figures that a comet must have a finite
life of only a few thousand years -- with possibly 10,000 years, but certainly
not millions or billions of years, as the upper limit (Van Flandern 1977).
It has been argued on this basis by J.H. Oort (1950), of the University
of Leiden, that somewhere out there in the darkness of space beyond our
solar system, there is a great cloud of comets, and that every so often
one is disturbed and enters the solar system, thus replenishing those that
are used up.[21] It
is admitted that there is not the slightest piece of evidence for this
fantasy (Brady 1970, 1064). Again, like Francis Crick's Panspermia theory
for the origin of life, an appeal is being made here to realms beyond man's
reach in order to safeguard the long ages required by the theory of evolution
(Noerdlinger 1977).
Meteorites, Tektites, and Moon Dust
On a clear night one can usually see a "shooting star", which nearly always seems to appear in the corner of the eye as a momentary streak of light among the stars, there for a second and then gone. In its orbit through space, the earth's atmosphere encounters a great many solid particles, most of which are of pinhead size. Occasionally, however, there is a larger piece. Upon entering our atmosphere at forty kilometers per second, the particles are very effectively burned up -- hence the streak of light -- and the oxides left form a fine dust that eventually settles to the earth (Moulton 1956, 59; Singer 1954). On rare occasions a large meteorite manages to survive in its passage through the atmosphere and land on the earth, where it often receives newspaper attention. These meteorites, when found, generally finish up in museums and have been extensively studied; they consist mostly of iron but contain some cobalt and approximately 2.5 percent nickel. It will be recalled from Chapter Four that while fossilization is claimed to be a rare event, it is nevertheless argued that the great number of fossils found in the sedimentary rocks is a result of the enormous spans of geological time available. By this same argument, then, it might be thought that although meteoritic impact on the earth's surface is a relatively rare event, nevertheless, because of the great spans of time available, the sedimentary rocks, should contain large numbers. The facts are, however, that not a single true meteorite has ever been found in the sedimentary rock record (Hindley 1977; Mason 1962, 4; Tarr 1932).
The mystery deepens when it is found that stoney meteorites that contain potassium compounds and, thus, allow dating by the potassium argon method, have reported ages of 4.6 billion years since solidification; that is, since they entered the earth's atmosphere. These stoney meteorites and tektites, which are small glassy beads of cosmic origin, are only found in recent deposits. Again, the tektites have been dated by the potassium/argon method and independently by the fission track method and have yielded ages ten times greater than expected by their position near the very top of the geologic column; this has given rise to a lot of controversy behind doors generally closed to the public (Gill 1970).[22] All this does not tell us the age of the earth but the absence of meteorites in the geologic column should lead us to question seriously the enormous spans of time claimed for the formation of all the sedimentary rocks. At the same time, the extreme ages claimed for the stoney meteorites and tektites lying within recent deposits should raise questions about the validity of the radiometric methods.
Returning now to the shooting stars and the meteoritic dust: Pettersson (1960), of the Swedish Oceanographic Institute, working on high mountain tops filtered measured quantities of air and analyzed the particles he found. Since the meteorites that have survived contain an average of 2.5 percent nickel, then the nickel content of the dust extracted represented that which came from meteors rather than from terrestrial sources. From a knowledge of the total volume of the earth's atmosphere, Pettersson reckoned that 14 million tons of meteoritic dust settled on the earth's surface each year; however, because of some variability in results, he concluded with a more conservative figure of five million tons (Pettersson 1960, 132). Isaac Asimov, the popular science writer, took the more liberal figure and concluded that at that rate, the dust piles up to about ten-millionths of an inch per year. This is certainly not much to get excited about. However, he then pointed out that over nearly five billion years, this would add up, if undisturbed, to a layer fifty-four feet deep over the entire surface of the earth (Asimov 1959, 35). Recalling that this dust is mostly iron and nickel oxides, it will be evident that no such layer or any trace of it is to be found; then, of course, it is argued that wind and water carried it all away and it is now in the ocean sediments.
Asimov, writing at about the time the Apollo moon landing was being planned, was reflecting a concern among scientists that in the absence of wind and rain a similar depth of dust would have accumulated on the moon's surface (Gold 1955; Lyttleton 1956).[23] There was before them the prospect that the Apollo lunar module would land only to disappear by slowly sinking into the moon dust! To avoid this very possibility, the lunar module was equipped with large pad feet. On 21 July 1969, more than 600 million people watched as television transmitted mankind's first footstep onto the moon's surface. Apollo 11 astronaut commander, Neil Armstrong's reply to Houston is worth quoting since the opening dialogue, reported by Wilford of The New York Times (21 July 1969:1), concerned the depth of the dust: "The surface is fine and powdery. I can pick it up loosely with my toe. It does adhere in fine layers like powdered charcoal to the sole and sides of my boots. I only go in a small fraction of an inch, maybe an eighth of an inch." As if to confirm this, astronauts Armstrong and Aldrin had great difficulty planting the American flag into the rocky and virtually dust-free ground, yet not one comment was made on the significance of the absence of the great depth of dust.
Pettersson (1950, 44) found meteorite spherules (microscopic spheres) in deep ocean sediments "millions of years old" so that they are recognized not to be a recent phenomenon; this leaves only two alternative explanations for the missing moon dust: either Pettersson and others were half a million times too high in their dust estimate, or there is something radically wrong with Asimov's five billion year assumption.
Before leaving the subject of the moon and the Apollo program, one of the experiments witnessed by television viewers during the moon walks was the installation of a small bank of mirrors facing the earth (Bender et al. 1973).[24] These were for the lunar laser-ranging experiments that have been carried out regularly since that time to measure the earth-moon distance to within a few centimeters. A large telescope on earth is aimed at the mirrors, a pulse of laser light sent out; the time interval between leaving and returning gives a measure of the distance. The laser-ranging experiments showed that the distance is increasing by about four centimeters per year -- nearly two inches (Stephenson 1982, 173). This is not only a remarkable testimony to the state of technological perfection achieved, but confirms and provides quantitative data for earlier theoretical work that predicted the separation as a result of the moon's gravitational pull on the oceans and the subsequent dissipation of energy as tidal friction; a further result of this same cause is a slowing of the rotation of the earth.
Jeffries, in 1929, recognized the possibility of calculating the age
of the earth-moon system from theoretical considerations of the dynamics
involved, but in the absence of real data it was necessary to make some
assumptions. As more information accumulated, however, it became possible
to get better estimates -- but then, even before the lunar laser-ranging
experiments were conducted, it was evident that serious difficulties were
being encountered. Baldwin explained the situation this way:
Jeffries' [1929] early studies of the effects of tidal friction yielded a rough age of the Moon of four billion years. Recently, however, Munk and MacDonald [1960] have interpreted the observations to indicate that tidal friction is a more important force than had realized and it would have taken not more than 1.78 billion years for tidal friction to drive the Moon outward to its present distance from any possible minimum distance. This period of time is so short, compared with the age of the earth, that serious doubts have been cast upon most proposed origins and histories of the moon (Baldwin 1965, 40).[25]
Hammond (1974), having the benefit of the laser-ranging data, concluded
that the current rate of separation of the earth-moon system implies an
initial separation of less than one billion years ago. Clearly, these times
are too short for the demands of evolution, and the method, once thought
to provide evidence for the long ages and earlier for George Darwin's fission
theory for the moon's origin, is now not likely to be seen in textbooks.
Nor are these same textbooks likely to make Baldwin's (1965, 42) candid
admission that science is at a loss adequately to explain the moon's origin,
but would, it seems, rather continue to promote outdated and thoroughly
discredited theories. All this lack of complete honesty, it may be recalled,
results from the most sacred of all precepts, bringing hasty excommunication
to any who would question its veracity -- namely, that the earth is 4.5
billion years old.
Earth's Decaying Magnetic Field
The earth is a magnet, and for a long time now navigation by compass
has made use of knowledge of this fact. There are, of course, north and
south poles, and just like the little bar magnets used for school instruction,
it is commonly believed that the interior of the earth consists of iron
or some mixture of iron and nickel. The core may be iron, but unlike the
bar magnet this is not the source of the magnetic field. It has been noted
earlier that the temperature increased 1°C every thirty meters (one
hundred feet) down into the earth, and, at this rate, by twenty-five kilometers
(sixteen miles) the temperature would be more than 750°C, which is
a red heat, and would continue hotter towards the earth's center (Thomson
1865).[26] A mere
eggshell thickness thus separates all life on earth from the terrible heat
beneath. At temperatures above their Curie temperature, that is above 750°C,
all magnetism in iron or magnetic iron ore is completely lost.[27]
It is evident, then, that the earth's magnet cannot be of the permanent
type, such as in a bar magnet, but must be the electromagnetic type and
function by huge electric currents surging around in the core.

Horace Lamb, 1849-1934. One of England's great scientists in his early fifties; his work on geomagnetism refuted the Darwinian demand for long ages and is seldom mentioned today. (Metropolitan Toronto Reference Library Board) |
The source of this electric current is unknown. Runcorn (1955) is a latter-day proponent of the theory that a dynamo, or electric power generator, operated by hypothetical movements of fluid in the earth's core, provides the current. However, mathematical analysis of the facts shows the dynamo theory to be totally inadequate as an explanation for the earth's magnetism (Cowling 1934). Horace Lamb provided a unique solution to the problem in 1883. He proposed that the electric current circulating within the earth is freely decaying; that is, its cessation has been retarded by self-induced currents created by the decay of the magnetic field. This effect may be experienced on a small scale when a radio continues to play for a second or so after its power has been disconnected. Lamb left open the questions of where the electrical power came from in the first place and when it was turned off, but at least the free decay he proposed is now well supported by more than 150 years of real magnetic data. Lamb's theory has not found the acceptance of orthodox science, even though it is just as plausible as hypothetical dynamos. One may suspect that the reason lies in the fact that, inadequate though it is, the dynamo theory offers an open-ended past, whereas Lamb's theory points to something abhorrent to many scientists, a relatively recent beginning (Jacobs 1967).[28] |
| The earth's magnetic field varies slightly from place to place and
can also vary slightly from day to day. In appreciation of this, Karl Gauss
(1834) organized magnetic measuring stations around the world and a method
to collect data that could be mathematically reduced to a single figure
representing the total strength of the earth's magnet.[29]
This value is the magnetic moment and was first recorded at the surprisingly
early date of 1835. Measurements have been made every few years since then,
and the published figures show a relatively rapid decay amounting to about
5 percent per one hundred years (McDonald and Gunst 1967, 1).[30]
The actual data from a U.S. government report is given in Appendix K. This
adequately confirms Lamb's theoretical work of a century ago and raises
the immediate question: When was the earth's electrical power shut off,
or when did magnetic decay begin?
FRONT: Karl Gauss, 1777-1855. REAR: Wilhelm Weber, 1804-91. Gauss the mathematician and Weber the physicist collaborated between 1831 and 1837 to organize the Magnetische Verein, which united a worldwide network of magnetic observatories; Europe alone had twenty-three stations. (Metropolitan Toronto Reference Library Board) |
 |
Barnes (1971) has analyzed the published data from 1835 to 1965 and concludes that the decay rate is exponential, with a half-life of only 1,400 years. An exponential decrease is normal for most natural processes and consists of an initial rapid decrease that becomes ever slower as it progresses. Half-life is just a convenient way of expressing a decay process that is theoretically never complete. More recent data from the Magsat geophysical exploration satellite shows that the overall intensity of the earth's magnetic field is declining at twenty-six nanoteslars per year, or has a half-life of a mere 830 years (Magsat down 1980).[31 ] This means that the magnetic field, which provides protection against cosmic radiation, is diminishing very rapidly and will be completely ineffective in a few thousand years. It also means that the magnetic field and the directly related electric currents must have been greater in the past, perhaps, double every 1,400 years, to use Barnes' estimate of half-life. There is an upper limit, however, because the circulating electric currents dissipate heat, and with double the current, the heat generated would be four times as great.[32] After only 50,000 years in the past, the heat generated in the core at that time would have been too great for life to have been possible on the surface; life over millions of years is out of the question. The most straightforward conclusion that can be drawn from the hard data (Appendix K) is that the decay of the magnetic field of the earth has an exponential relationship, and that from the Joule heating effect mentioned, the decay is not likely to have begun more than about 10,000 years ago. Evidence from other natural processes would indicate that this was coincidental with the earth's beginning. Suppose we acknowledge the fact that extrapolation is a hazardous business. Then even if the beginning point on the decay curve is off by two orders of magnitude -- a virtual impossibility -- the beginning is merely set back to one million years. This is far too short a time for the guardians of Lyell's uniformitarianism; it is not surprising that Barnes' analysis of the data has been totally rejected, as has the earlier work of Lamb. However, the facts remain, and an ingenious escape has been found, similar in principle to the argument used to refute the evidence of the shrinking sun.
When hot molten rocks (magma) containing iron oxides cool below the
Curie temperature, they become magnetized by the earth's magnetic field
and so preserve within their mass the earth's magnetic intensity and pole
direction at that time. A massive body of data collected in recent years
from rock units around the world now show that the earth's magnetic field
has reversed its polarity at least fifty times in the past. These reversals
were not changes in the earth's rotation or gravity but simply 180°
changes in the direction a compass needle would point. The last reversal
seems to have occurred some thousands of years ago. The reversals are found
at different levels in the geologic strata and, according to the evolutionary
interpretation of these strata, it is believed that the process takes hundreds
of thousands of years. At the 1986 International Conference on Creationism,
D. R. Humphreys proposed a mechanism based upon convection currents to
explain how rapid reversals can take place then proposed that most of these
reversals occurred during the twelve months of the Genesis Flood; he retained
Lamb's proposal that the overall energy of the earth's magnetic field has
been decreasing throughout earth's history. Humphreys suggested that support
for his hypothesis would be to find a thin lava flow which would have cooled
through the Curie point relatively quickly but, nevertheless, have recorded
a full 180° reversal. (See: D. R. Humphreys Creation Research Society
Quarterly Vol. 26, March 1990, p.132). In 1989 two respected paleomagnetists,
R. Coe and M. Prévot, reported precisely this evidence, first of
one reversal, then later in 1991, discovered a second reversal in a succeeding
lava flow at the same location! These reversals had each been completed
within two weeks during the time the lava flow was cooling. The rock unit
was a Pliocene basalt flow at Steens Mountain, Oregon. (See: R. Coe Nature
Vol.
374, April 20, 1995, p.687-92.) Coe and Prévot ascribed this extremely
rapid change in geomagnetic field as originating in the earth's core and
confessed that the rapidity of this change "truly strains the imagination".
The creation explanation relies upon Lamb's freely decaying magnetic field
with reversals occurring during the Flood. The evolutionary explanation
demands a dynamo seemingly generating power for ever and reversing for
unknown reasons. (Carrigan and Gubbins 1979).[33]
The Missing Radiogenic Helium
During the radioactive decay of uranium and thorium in the earth's crust,
alpha particles are given off, and these become helium 4, the most abundant
isotope of helium. A glance at Appendix C will show that a total of eight
alpha particles are produced as each uranium 238 atom decays to lead 206.
Estimates have been made of the total uranium and thorium in the earth's
surface, and from this the rate of production of helium is reckoned to
be 3 x 109 grams per year. In addition to this, about
the same quantity of helium is generated each year in the upper atmosphere
by the bombardment of cosmic rays. If helium 4 has been released into our
atmosphere at this rate for some four billion years, then the total quantity
of helium 4 present today should be about 1020 grams.
In fact, the actual quantity found is a thousand times less than this figure,
which would indicate that the earth is only a few million years old.[34]
More recently, Vardiman has shown that the earth's atmosphere contains
only 0.04% of the radiogenic helium it should contain if the earth were
really billions of years old (Vardiman 1990). The immediate reaction is
to suppose that since helium is a light gas, it has been lost from our
atmosphere to outer space. This is not, however, necessarily so and it
appears that our atmosphere has more likely gained helium from space. It
turns out that atmospheric helium consists of a mixture of the isotopes
helium 3 and helium 4 in a certain ratio, whereas the ratio of helium 3
to helium 4 in the earth's crust is ten times less. If helium was being
lost to space, both isotopes would go at the same rate, and the ratio would
have remained constant. The evidence of the difference indicates that the
ratio must rather have been increasing from that in the rocks to that in
the atmosphere by a factor of ten times to reach its present value. Taking
the difference in the two ratios, Cook (1957), writing a cautiously worded
letter to Nature, has concluded that helium 3 must have been added
to our atmosphere. That being so, he then points out the process could
have begun not more than ten thousand years ago. As a final word to this
section, some work carried out by the Institute of Creation Research, California,
and reported at the time this section was being up-dated (2003), has shown
that the radiogenic helium gas is still largely present in the zircon crystals.
This startling evidence not only explains the whereabouts of the missing
radiogenic helium but clearly indicates that there has been insufficient
time for diffusion to the atmosphere to occur.

When opened after thirty-three years of disuse, a tunnel bored through London clay was found to contain stalactites or dripstone more than twenty-four inches long. (The Times, London) |
Stalactites or Stalagmites?
Returning to terra firma, there are, in almost every country, limestone caverns with intriguing names, such as the Dragon Caves (Island of Majorca), that capture the tourists' attention. Carlsbad Caverns, New Mexico, are probably the best known in North America (Sutherland 1953).[35] Typically, the visitor receives for his entrance fee a printed tract and a guided tour, in which he is assured that the beautiful floodlit cave formations have taken millions of years to reach their present size. Stalactites, incidentally, are the ones that hang downwards. When water runs through limestone, it dissolves some of this mineral. As the mineral-laden water hangs as a drop from a crack in the cave roof, it is exposed to the air, where the water evaporates and leaves the mineral deposit. How long does all this take, drip by drip? In underground vaults and tunnels, stalactites, in their initial stages, can usually be found and are known as "dripstone". Dripstone can grow to appreciable lengths in just a few years if left undisturbed and may even be seen an inch or two long in underground railway stations that are in daily use. The photograph shows dripstone as minor stalactites more than sixty centimeters (twenty-four inches) long in a London tunnel, disused since its days as an air-raid shelter, 1941-45. They have grown this length in only thirty-three years, and little imagination is required to picture their growth after just a hundred times this number of years. Five thousand years is a more reasonable age for the limestone caverns, but the little tracts ask us to believe it has taken a thousand times longer. |
Very High Pressure Oil Wells
When drilling for oil and gas, the drill passes through solid rock for thousands of feet, and well drillers have become accustomed to increasing pressure with depth at the rate of about a half pound per square inch per foot depth, so that at 10,000 feet, the pressure is 5,000 per square inch. Fairly massive equipment is required to handle these pressures, but, occasionally, a zone is encountered where the pressure more than doubles, causing difficulty and some danger in the drilling operation. In these circumstances, the drill passes from a zone of high pressure to an adjacent zone of exceptionally high pressure, and structural geologists have wondered how it is possible for such great pressure differences to have existed side by side for "scores of millions of years" (Dickey et al. 1968).[36] The broader question might also be asked: How can oil or gases remain under great pressure for millions of years without dissipation and leaking through to the surface? From what has already been said in this and the previous chapter, the millions of years that are taken for granted should be the first area open to question.
After all, the reported ages of the oil and gas by the C14
method were only a few thousand years, yet these results are usually dismissed,
not for actual technical reasons, but because they do not meet the expectations
of a much greater length of time. The whole area of preconception and presumption
in science is not merely of academic interest, since the assumed age and
stability of rock units directly influence such issues as the storage of
nuclear wastes. The high pressures in oil and gas wells are, rather, evidence
for a youthful age, indicating, perhaps, thousands of years -- rather than
millions of years -- for the rock units.
Population Explosion
Government policy makers are always on the horns of a dilemma when it comes to the question of human population. On the one hand, they warn against population increase in foreign countries because of the extra mouths to feed, but on the other hand, they like to encourage an increase in their own country because the babes of today become the taxpayers of tomorrow. By the time the problem gets to the United Nations, it becomes a very confused matter of wheat deals, family taxation or subsidy, and contraceptive devices. No one can be certain of the number of people in the world, and the figures, particularly from the underdeveloped countries, are largely estimates. Nevertheless, there is no doubt that, even though the reproduction rate per family is relatively low today, the overall number of people in the world is greater than it has ever been, and that it is continuing to grow. A generation ago, the writings of William Vogt (1948) stirred the imagination of Dixie-cup king Hugh Moore, who flooded America with alarmist and blatantly untruthful literature intended to curb the population increase. Moore died in 1972 and so, it seems, did the campaign, but the United Nations has continued to play its own low-key, but steady, part in bringing the world's population growth to zero.[37]
Demographers earn a living by juggling with the figures of population obtained in the national census. By the use of mathematical formulas, they can usefully predict, for example, when and where to build schools for tomorrow's students. There are a number of formulas, all of which tend to give roughly the same result but with various degrees of refinement (a rather simple one is given in Appendix L). Of particular interest, however, is the fact that these formulas can be used to find populations in the past as well as in the future (for examples see Appendix L).
If humanity is really 3.5 million years old, or whatever the latest Leakey/Johanson debate has decided, then today's world population can be predicted by use of the formula and selecting likely data. For example, with a modest estimate of 2.2 children per average family, an equally modest average generation life span of twenty years, and parents never living long enough to see their grandchildren, then the world population would have grown from a single family to 102070 (one followed by 2,070 zeroes!) people alive at the same time at the end of the first million years. This number is so large that our entire universe could contain only a small fraction of them, stacked shoulder to shoulder!
The use of formulas gives the maximum figure possible from the variables
that have been selected, and it is cogently argued that natural disasters
have always played a hand in keeping human population in check; the long-term
picture is thus seen to be one of population stability. History shows,
for example, that the Justinian plague, A.D. 540-90, took 100 million lives;
the Black Death, A.D. 1348-80, swept away 150 million from Europe alone;
and even as late as 1918-19, the influenza epidemic took 25 million lives
(Wallace 1969; Webster 1799). Four things need to be kept in mind (it might
also help to practice with a computer or large calculator to get a "feel"
for the way populations grow). First, so far as they go, historical records
show that families did not stop at 2.2 children, but many, it seems, approached
the biological maximum. About half survived, and there were consequently
four or five left to reproduce in the next generation. Second, the picture
of ancient man scratching a bare existence in caves is nineteenth century
speculation. The very earliest remains of human habitation, as at Mohenjo-daro
in India, for example, have been found with the lowest strata showing a
more advanced city civilization in the fourth millenium B.C. than more
recent occupation levels (Durant 1:395). Third, no matter how infrequent
fossilization of human or animal remains may claim to be, the fossil record
simply does not support the millions upon millions of creatures that would
have existed over the vast ages required. And fourth, the awful figures
for natural disasters are very quickly made up for by the subsequent rates
of increase among the survivors (Langer 1964).[38]
It is difficult to imagine how reproduction and disaster can have kept
such a delicate balance for a million years or more among the human population.
Zero population growth occurs when there is an average of 2.00 children
per family, but even the slightest increase, for example, from 2.01 to
2.02 children per family, causes a tremendous difference over the evolutionary
time scales proposed. When textbook authors, such as Stansfield (1977,
82), acknowledge the population problem at all, they explain it away by
speaking of "population stability". However, what is really meant is "population
oscillation near the zero growth level", and this, it may be recognized,
is the same strategem used to refute the shrinking sun and the decay of
the earth's magnetic field.[39]
On the other hand, if the young earth can be accepted, then the world's
population today would be almost exactly what would be expected from the
four couples surviving the Genesis Flood some five thousand years ago and
would take into account all the natural disasters. Details are given in
Appendix L.
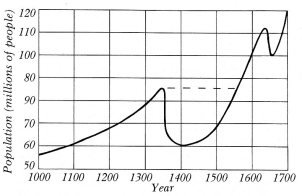
Recovery of European population following the plagues of 1347 was only two hundred years -- an insignificant moment in the evolutionary time scale. (After Langer 1964; author) |
These two chapters have dealt with the important issue of time and, specifically, the age of the earth. In summary, a number of examples have been given of natural processes that are well documented and for which science has no adequate explanation, in terms of an old earth. While some of these processes indicate an age of millions and others of thousands of years, no single process yet gives an exact age. When taken together, however, an age of less than 10,000 years fits all the facts most reasonably. There are many other natural processes, all of which indicate a young earth -- that is, at least a thousand times less than the current estimate of 4.5 thousand million years. In contrast, the evidence for the old earth model hangs almost exclusively on the assumptions inherent within the radiometric methods. Even then, the C14 method must be excluded, since it supports the young earth model. There will probably never be proof for either model, and it becomes a matter of personal choice for each individual to adopt the model that best seems to fit all the facts. In the next chapter, some of the mental contortions to try to reconcile the young earth/old earth debate will be shown, together with a little insight into the characters responsible for these ideas being still very much with us. |
|
|
| << PREV |
|